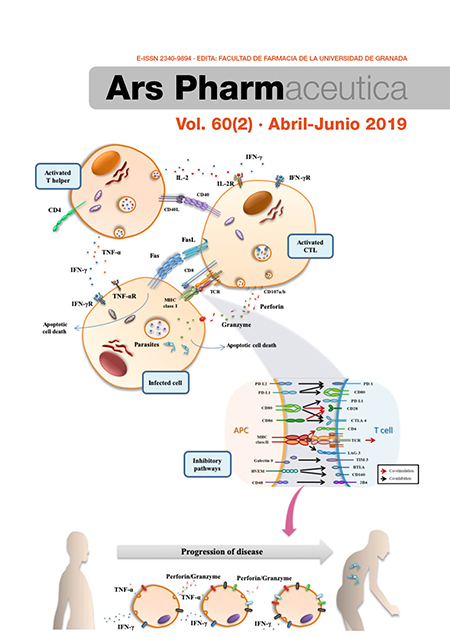
T-cell exhaustion process during chronic infection caused by intracellular trypanosomatids
DOI:
https://doi.org/10.30827/ars.v60i2.9432Keywords:
Chagas disease, leishmaniasis, T-cell exhaustion process, inhibitory receptors, cytokines.Abstract
Two of the most important neglected tropical diseases, Chagas disease and leishmaniasis, are caused by protozoan intracellular parasites of the Trypanosomatida order. These infections provoke a high social burden and lead to the death of a large number of patients. The host triggers several immune mechanisms, but in the absence of adequate treatment, the infection becomes chronic and in many cases causes the appearance of serious alterations. T lymphocytes are fundamental cells of the adaptive system and are the main immune elements that orchestrate the cell-to-cell response in the context of intracellular infections. Furthermore, it has been described that continuous and persistent stimulation in response to pathogenic antigens causes loss of antigen-specific functional capacities in the T cell subsets. This process is known as exhaustion. This review explores the results to date of the exhaustion process during chronic infections caused by the trypanosomatid parasites Leishmania spp. and Trypanosoma cruzi. A large amount of evidence shows upregulation of the markers of the exhaustion process, namely, the inhibitory receptors, during these chronic infections. This increased expression is observed in both the CD4+ and CD8+ T cell populations. In parallel, with this increased expression of inhibitory receptors, the loss of antigen-specific functional capacity of these T cells is detected, reducing the lymphoproliferative potential and the ability to produce protective molecules against these parasitic infections, such as Th1-like cytokines, among others. Additionally, a positive correlation between the high coexpression of these inhibitory molecules and the severity of the pathology is demonstrated. Furthermore, T cell populations experience a phenotypic fluctuation in the course of these infections toward the predominance of effector memory subsets with a late or terminal differentiation state. This balancing in turn affects the functional capacity of the T cells and enriches the number of cells with senescent and apoptotic characteristics. Thus, it has been demonstrated the existence of an exhaustion process that affects key populations for the parasite control. However, the role of this process in the progression of the severity of these pathologies is still unknown.
The current drugs used to treat these neglected diseases seem to partially reverse this exhaustion process, denoting a reduction in the high inhibitory receptor expression observed prior to chemotherapies. An improvement in the functional capacity of these T cell populations is also observed, which could be related to the reversion of the dysfunctional process. However, the efforts made to date to evaluate blocking therapies do not lead us to a promising conclusion. It will probably be necessary to test the simultaneous blockade of several pathways and to continue advancing the knowledge to verify their possible use as immunotherapy. It is therefore necessary to continue investigating how this process is triggered and to what extent it influences the appearance of the symptomatology of patients.
Downloads
References
Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175-96. doi: 10.1093/bmb/lds031.
Mortality GBD, Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459-544. doi: 10.1016/S0140-6736(16)31012-1.
Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305-18. doi: 10.1016/j.cimid.2004.03.004.
Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115(1-2):14-21. doi: 10.1016/j.actatropica.2009.11.003.
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. 2017;6:750. doi: 10.12688/f1000research.11120.1.
Bern C. Chagas’ Disease. N Engl J Med. 2015;373(19):1882. doi: 10.1056/NEJMc1510996.
de Morais CG, Castro Lima AK, Terra R, dos Santos RF, Da-Silva SA, Dutra PM. The Dialogue of the Host-Parasite Relationship: Leishmania spp. and Trypanosoma cruzi Infection. Biomed Res Int. 2015;2015:324915. doi: 10.1155/2015/324915.
Padilla AM, Bustamante JM, Tarleton RL. CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol. 2009;21(4):385-90. doi: 10.1016/j.coi.2009.07.006.
Ruiz JH, Becker I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Parasite Immunol. 2007;29(12):671-8. doi: 10.1111/j.1365-3024.2007.00991.x.
Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179(12):8243-51.
Dominguez MR, Silveira EL, de Vasconcelos JR, de Alencar BC, Machado AV, Bruna-Romero O, et al. Subdominant/cryptic CD8 T cell epitopes contribute to resistance against experimental infection with a human protozoan parasite. PloS one. 2011;6(7):e22011. doi: 10.1371/journal.pone.0022011.
Krawczyk CM, Shen H, Pearce EJ. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect Immun. 2007;75(7):3556-60. doi: 10.1128/IAI.00086-07.
Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun. 2009;384(4):405-8. doi: 10.1016/j.bbrc.2009.04.134.
Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. Indian J Med Res. 2004;119(6):238-58.
Rodrigues MM, Ribeirao M, Boscardin SB. CD4 Th1 but not Th2 clones efficiently activate macrophages to eliminate Trypanosoma cruzi through a nitric oxide dependent mechanism. Immunol Lett. 2000;73(1):43-50.
Tsagozis P, Karagouni E, Dotsika E. CD8(+) T cells with parasite-specific cytotoxic activity and a Tc1 profile of cytokine and chemokine secretion develop in experimental visceral leishmaniasis. Parasite Immunol. 2003;25(11-12):569-79. doi: 10.1111/j.0141-9838.2004.00672.x.
von Stebut E, Udey MC. Requirements for Th1-dependent immunity against infection with Leishmania major. Microbes Infect. 2004;6(12):1102-9. doi: 10.1016/j.micinf.2004.05.024.
Wizel B, Nunes M, Tarleton RL. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J Immunol 1997;159(12):6120-30.
Goncalves-de-Albuquerque SDC, Pessoa ESR, Trajano-Silva LAM, de Goes TC, de Morais RCS, da COCN, et al. The Equivocal Role of Th17 Cells and Neutrophils on Immunopathogenesis of Leishmaniasis. Front Immunol. 2017;8:1437. doi: 10.3389/fimmu.2017.01437.
Magalhaes LM, Villani FN, Nunes Mdo C, Gollob KJ, Rocha MO, Dutra WO. High interleukin 17 expression is correlated with better cardiac function in human Chagas disease. J Infect Dis. 2013;207(4):661-5. doi: 10.1093/infdis/jis724.
Sousa GR, Gomes JA, Damasio MP, Nunes MC, Costa HS, Medeiros NI, et al. The role of interleukin 17-mediated immune response in Chagas disease: High level is correlated with better left ventricular function. PloS one. 2017;12(3):e0172833. doi: 10.1371/journal.pone.0172833.
Bunn PT, Montes de Oca M, de Labastida Rivera F, Kumar R, Ng SS, Edwards CL, et al. Distinct Roles for CD4(+) Foxp3(+) Regulatory T Cells and IL-10-Mediated Immunoregulatory Mechanisms during Experimental Visceral Leishmaniasis Caused by Leishmania donovani. J immunol. 2018. doi: 10.4049/jimmunol.1701582.
de Araujo FF, Vitelli-Avelar DM, Teixeira-Carvalho A, Antas PR, Assis Silva Gomes J, Sathler-Avelar R, et al. Regulatory T cells phenotype in different clinical forms of Chagas’ disease. PLoS Negl Trop Dis. 2011;5(5):e992. doi: 10.1371/journal.pntd.0000992.
Gigley JP, Bhadra R, Moretto MM, Khan IA. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28(9):377-84. doi: 10.1016/j.pt.2012.07.001.
Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J immunol. 2012;188(7):2957-65. doi: 10.4049/jimmunol.1100038.
Ivashkiv LB. How ITAMs inhibit signaling. Sci Signal. 2011;4(169):pe20. doi: 10.1126/scisignal.2001917.
Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11(5):603-13.
Fuertes Marraco SA, Neubert NJ, Verdeil G, Speiser DE. Inhibitory Receptors Beyond T Cell Exhaustion. Front Immunol. 2015;6:310. doi: 10.3389/fimmu.2015.00310.
Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21(2):179-86. doi: 10.1016/j.coi.2009.01.010.
Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory Receptor Expression Depends More Dominantly on Differentiation and Activation than “Exhaustion” of Human CD8 T Cells. Front Immunol. 2013;4:455. doi: 10.3389/fimmu.2013.00455.
Attanasio J, Wherry EJ. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity. 2016;44(5):1052-68. doi: 10.1016/j.immuni.2016.04.022.
Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15(8):486-99. doi: 10.1038/nri3862.
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29-37. doi: 10.1038/ni.1679.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492-9.
Kahan SM, Wherry EJ, Zajac AJ. T cell exhaustion during persistent viral infections. Virology. 2015;479-480:180-93. doi: 10.1016/j.virol.2014.12.033.
Rodrigues V, Cordeiro-da-Silva A, Laforge M, Ouaissi A, Akharid K, Silvestre R, et al. Impairment of T cell function in parasitic infections. PLoS Negl Trop Dis. 2014;8(2):e2567. doi: 10.1371/journal.pntd.0002567.
Medina-Colorado AA, Osorio EY, Saldarriaga OA, Travi BL, Kong F, Spratt H, et al. Splenic CD4+ T Cells in Progressive Visceral Leishmaniasis Show a Mixed Effector-Regulatory Phenotype and Impair Macrophage Effector Function through Inhibitory Receptor Expression. PloS one. 2017;12(1):e0169496. doi: 10.1371/journal.pone.0169496.
Schaut RG, Grinnage-Pulley TL, Esch KJ, Toepp AJ, Duthie MS, Howard RF, et al. Recovery of antigen-specific T cell responses from dogs infected with Leishmania (L.) infantum by use of vaccine associated TLR-agonist adjuvant. Vaccine. 2016;34(44):5225-34. doi: 10.1016/j.vaccine.2016.09.016.
Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J immunol. 2013;191(11):5542-50. doi: 10.4049/jimmunol.1301810.
Habib S, El Andaloussi A, Elmasry K, Handoussa A, Azab M, Elsawey A, et al. PDL-1 Blockade Prevents T Cell Exhaustion, Inhibits Autophagy, and Promotes Clearance of Leishmania donovani. Infect Immun. 2018;86(6). doi: 10.1128/IAI.00019-18.
Chiku VM, Silva KL, de Almeida BF, Venturin GL, Leal AA, de Martini CC, et al. PD-1 function in apoptosis of T lymphocytes in canine visceral leishmaniasis. Immunobiology. 2016;221(8):879-88. doi: 10.1016/j.imbio.2016.03.007.
Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, et al. CD8 T cell exhaustion in human visceral leishmaniasis. J Infect Dis. 2014;209(2):290-9. doi: 10.1093/infdis/jit401.
Egui A, Ledesma D, Perez-Anton E, Montoya A, Gomez I, Robledo SM, et al. Phenotypic and Functional Profiles of Antigen-Specific CD4(+) and CD8(+) T Cells Associated With Infection Control in Patients With Cutaneous Leishmaniasis. Front Cell Infect Microbiol. 2018;8:393. doi: 10.3389/fcimb.2018.00393.
Hernandez-Ruiz J, Salaiza-Suazo N, Carrada G, Escoto S, Ruiz-Remigio A, Rosenstein Y, et al. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4(11):e871. doi: 10.1371/journal.pntd.0000871.
Arguello RJ, Albareda MC, Alvarez MG, Bertocchi G, Armenti AH, Vigliano C, et al. Inhibitory receptors are expressed by Trypanosoma cruzi-specific effector T cells and in hearts of subjects with chronic Chagas disease. PloS one. 2012;7(5):e35966. doi: 10.1371/journal.pone.0035966.
Lasso P, Mateus J, Pavia P, Rosas F, Roa N, Thomas MC, et al. Inhibitory Receptor Expression on CD8+ T Cells Is Linked to Functional Responses against Trypanosoma cruzi Antigens in Chronic Chagasic Patients. J immunol. 2015;195(8):3748-58. doi: 10.4049/jimmunol.1500459.
Perez-Anton E, Egui A, Thomas MC, Puerta CJ, Gonzalez JM, Cuellar A, et al. Impact of benznidazole treatment on the functional response of Trypanosoma cruzi antigen-specific CD4+CD8+ T cells in chronic Chagas disease patients. PLoS Negl Trop Dis. 2018;12(5):e0006480. doi: 10.1371/journal.pntd.0006480.
Pack AD, Collins MH, Rosenberg CS, Tarleton RL. Highly competent, non-exhausted CD8+ T cells continue to tightly control pathogen load throughout chronic Trypanosoma cruzi infection. PLoS Pathog. 2018;14(11):e1007410. doi: 10.1371/journal.ppat.1007410.
Gutierrez FR, Mariano FS, Oliveira CJ, Pavanelli WR, Guedes PM, Silva GK, et al. Regulation of Trypanosoma cruzi-induced myocarditis by programmed death cell receptor 1. Infect Immun. 2011;79(5):1873-81. doi: 10.1128/IAI.01047-10.
Fonseca R, Salgado RM, Borges da Silva H, do Nascimento RS, D’Imperio-Lima MR, Alvarez JM. Programmed Cell Death Protein 1-PDL1 Interaction Prevents Heart Damage in Chronic Trypanosoma cruzi Infection. Front Immunol. 2018;9:997. doi: 10.3389/fimmu.2018.00997.
Arguello RJ, Vigliano C, Cabeza-Meckert P, Viotti R, Garelli F, Favaloro LE, et al. Presence of antigen-experienced T cells with low grade of differentiation and proliferative potential in chronic Chagas disease myocarditis. PLoS Negl Trop Dis. 2014;8(8):e2989. doi: 10.1371/journal.pntd.0002989.
Albareda MC, Olivera GC, Laucella SA, Alvarez MG, Fernandez ER, Lococo B, et al. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J immunol. 2009;183(6):4103-8. doi: 10.4049/jimmunol.0900852.
Parodi C, Garcia Bustos MF, Barrio A, Ramos F, Gonzalez Prieto AG, Mora MC, et al. American tegumentary leishmaniasis: T-cell differentiation profile of cutaneous and mucosal forms-co-infection with Trypanosoma cruzi. Med Microbiol Immunol. 2016;205(4):353-69. doi: 10.1007/s00430-016-0455-0.
Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, et al. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85(1):88-97. doi: 10.1189/jlb.0208107.
Dunne PJ, Belaramani L, Fletcher JM, Fernandez de Mattos S, Lawrenz M, Soares MV, et al. Quiescence and functional reprogramming of Epstein-Barr virus (EBV)-specific CD8+ T cells during persistent infection. Blood. 2005;106(2):558-65. doi: 10.1182/blood-2004-11-4469.
Xu W, Larbi A. Markers of T Cell Senescence in Humans. Int J Mol Sci. 2017;18(8). doi: 10.3390/ijms18081742.
Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, Tarleton RL, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas’ disease patients. Int immunol. 2006;18(3):465-71. doi: 10.1093/intimm/dxh387.
Albareda MC, Olivera GC, De Rissio AM, Postan M. Assessment of CD8(+) T cell differentiation in Trypanosoma cruzi-infected children. Am J Trop Med Hyg. 2010;82(5):861-4. doi: 10.4269/ajtmh.2010.09-0604.
Mateus J, Lasso P, Pavia P, Rosas F, Roa N, Valencia-Hernandez CA, et al. Low frequency of circulating CD8+ T stem cell memory cells in chronic chagasic patients with severe forms of the disease. PLoS Negl Trop Dis. 2015;9(1):e3432. doi: 10.1371/journal.pntd.0003432.
Fiuza JA, Fujiwara RT, Gomes JA, Rocha MO, Chaves AT, de Araujo FF, et al. Profile of central and effector memory T cells in the progression of chronic human chagas disease. PLoS Negl Trop Dis. 2009;3(9):e512. doi: 10.1371/journal.pntd.0000512.
Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290-7. doi: 10.1038/nm.2446.
Clarencio J, de Oliveira CI, Favali C, Medina O, Caldas A, Costa CH, et al. Could the lower frequency of CD8+CD18+CD45RO+ lymphocytes be biomarkers of human VL? Int Immunol. 2009;21(2):137-44. doi: 10.1093/intimm/dxn131.
Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911-27.
Cunha CF, Ferraz R, Pimentel MI, Lyra MR, Schubach AO, Da-Cruz AM, et al. Cytotoxic cell involvement in human cutaneous leishmaniasis: assessments in active disease, under therapy and after clinical cure. Parasite Immunol. 2016;38(4):244-54. doi: 10.1111/pim.12312.
Campos TM, Costa R, Passos S, Carvalho LP. Cytotoxic activity in cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2017;112(11):733-40. doi: 10.1590/0074-02760170109.
Santos Cda S, Boaventura V, Ribeiro Cardoso C, Tavares N, Lordelo MJ, Noronha A, et al. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNgamma(+)-mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol. 2013;133(6):1533-40. doi: 10.1038/jid.2013.4.
Mateus J, Perez-Anton E, Lasso P, Egui A, Roa N, Carrilero B, et al. Antiparasitic Treatment Induces an Improved CD8(+) T Cell Response in Chronic Chagasic Patients. J immunol. 2017;198(8):3170-80. doi: 10.4049/jimmunol.1602095.
Sathler-Avelar R, Vitelli-Avelar DM, Eloi-Santos SM, Gontijo ED, Teixeira-Carvalho A, Martins-Filho OA. Blood leukocytes from benznidazole-treated indeterminate chagas disease patients display an overall type-1-modulated cytokine profile upon short-term in vitro stimulation with Trypanosoma cruzi antigens. BMC Infect Dis. 2012;12:123. doi: 10.1186/1471-2334-12-123.
Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, Borges JD, Lana M, Teixeira-Carvalho A, et al. Benznidazole treatment during early-indeterminate Chagas’ disease shifted the cytokine expression by innate and adaptive immunity cells toward a type 1-modulated immune profile. Scand J Immunol. 2006;64(5):554-63. doi: 10.1111/j.1365-3083.2006.01843.x.
Vallejo A, Monge-Maillo B, Gutierrez C, Norman FF, Lopez-Velez R, Perez-Molina JA. Changes in the immune response after treatment with benznidazole versus no treatment in patients with chronic indeterminate Chagas disease. Acta trop. 2016;164:117-24. doi: 10.1016/j.actatropica.2016.09.010.
Bertocchi GL, Vigliano CA, Lococo BG, Petti MA, Viotti RJ. Clinical characteristics and outcome of 107 adult patients with chronic Chagas disease and parasitological cure criteria. Trans R Soc Trop Med Hyg. 2013;107(6):372-6. doi: 10.1093/trstmh/trt029.
Fragata-Filho AA, Franca FF, Fragata Cda S, Lourenco AM, Faccini CC, Costa CA. Evaluation of Parasiticide Treatment with Benznidazol in the Electrocardiographic, Clinical, and Serological Evolution of Chagas Disease. PLoS Negl Trop Dis. 2016;10(3):e0004508. doi: 10.1371/journal.pntd.0004508.
Machado-de-Assis GF, Silva AR, Do Bem VA, Bahia MT, Martins-Filho OA, Dias JC, et al. Posttherapeutic cure criteria in Chagas’ disease: conventional serology followed by supplementary serological, parasitological, and molecular tests. Clin Vaccine Immunol. 2012;19(8):1283-91. doi: 10.1128/CVI.00274-12.
Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Jr., Rosas F, et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N Engl J Med 2015;373(14):1295-306. doi: 10.1056/NEJMoa1507574.
Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5(5):e1000431. doi: 10.1371/journal.ppat.1000431.
Filippis C, Arens K, Noubissi Nzeteu GA, Reichmann G, Waibler Z, Crauwels P, et al. Nivolumab Enhances In Vitro Effector Functions of PD-1(+) T-Lymphocytes and Leishmania-Infected Human Myeloid Cells in a Host Cell-Dependent Manner. Front Immunol. 2017;8:1880. doi: 10.3389/fimmu.2017.01880.
Published
How to Cite
Issue
Section
License
Copyright (c) 2019 Manuel Carlos Lopez Lopez, Elena Pérez-Antón, M. Carmen Thomas

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The articles, which are published in this journal, are subject to the following terms in relation to the rights of patrimonial or exploitation:
- The authors will keep their copyright and guarantee to the journal the right of first publication of their work, which will be distributed with a Creative Commons BY-NC-SA 4.0 license that allows third parties to reuse the work whenever its author, quote the original source and do not make commercial use of it.
b. The authors may adopt other non-exclusive licensing agreements for the distribution of the published version of the work (e.g., deposit it in an institutional telematic file or publish it in a monographic volume) provided that the original source of its publication is indicated.
c. Authors are allowed and advised to disseminate their work through the Internet (e.g. in institutional repositories or on their website) before and during the submission process, which can produce interesting exchanges and increase citations of the published work. (See The effect of open access).